The pressure decrease test
At the pressure decrease test the test object is filled with a gas, in most cases pressurized air, to a pressure higher than atmospheric pressure. If a leak is present, the pressure will decrease over time. From the pressure difference between start and end of the test, multiplied with the volume of the test object and divided by the measuring time the leak rate can be calculated. 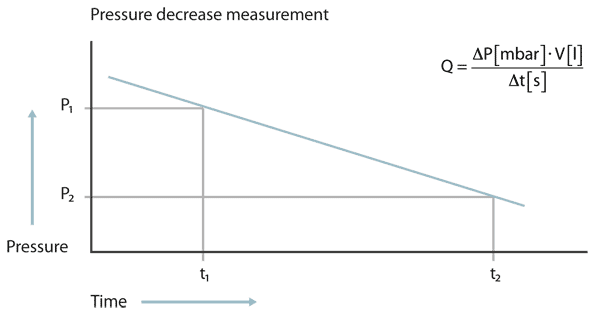
At the pressure decrease test the pressure must be measured with high accuracy and the temperature must be kept constant. Even a change of temperature of a few Centigrade can produce a pressure change larger than that from a leak
When the gas filling pressure is higher more than several bar above atmospheric pressure, a strength test of the container must be performed. This firmness test is done in most cases with water filling. The container is filled with water until no air is remaining. Then the pressure is increased in most cases to factor 1.3 higher pressure than the normal operating pressure of the container. If during this test a crack would happen only some water would be spilled. If the container would have been filled with gas, then the container can explode and make serious damage and injuries or even danger of life to human people.
Such a water pressure test can also work as a rough leak test. A small amount of water coming out of the container will make a distinct pressure change. Also during this test a change of temperature produces a change of pressure because the thermal expansion coefficient of water is larger than that of steel. After such a strength test the container must become completely dry before performing the leak test with gas filling. A small leak can block the gas throughput because water sticks inside the capillary because of its surface tension.
In general it can be said that this leak test procedure is good for small test objects. Objects with large volumes or large geometrical dimensions have easily failures in measurement mostly because of temperature changes It is possible that a large test object does have different temperatures on each end. If someone seriously has to plan such a test, he can contact me for discussion of failure calculations.


