What is Pressure?
Pressure is defined as force per unit surface area. Where does this force originate from in a container filled with gas? Gas consists of a large number of particles (atoms, molecules) which are permanently in motion. When they collide with a surface, their recoil produces a force. Pressure is the sum of these forces produced by particles on unit surface area. The unit, in which the pressure is measured, is the Pascal (Pa = N/m2, Newton per square meter). Another valid unit of pressure is the bar (1 bar = 105 Pa). The standard atmospheric pressure (dry air at sea level) is 1.013 bar. This value comes from the older, and no longer used unit the Torr (760 Torr = 760 mm mercury column).
What is Vacuum?
Vacuum is produced by removing gas from a container. It is not possible to remove all the gas from a container by pumping; particles still remain in the container, producing a pressure. Zero pressure is therefore not possible in practice. The pressure relative to the absolute vacuum is called the absolute pressure. Thus there is still a positive pressure even in a good vacuum. Atmospheric pressure is about 1 bar absolute, and manometers which zero at atmospheric pressure and show negative in vacuum, measure a relative pressure against atmospheric pressure. Note that the negative values do not represent a negative absolute pressure.
A gas having a negative pressure relative to the atmosphere is called vacuum. By definition there are four levels of vacuum, depending on the size of the negative relative pressure:
Composition of Air
Air is a mixture of different gases. More than 99% of air consist of nitrogen and oxygen.
The rest are other gases.
Table 1
What is Partial Pressure?
Partial pressure is produced by a single gas in a mixture of gases. The table below shows the percentage composition of air. At a pressure of 1.013 bar the oxygen content is 21.224%, the partial pressure of oxygen in air is = 0.21224 bar or 212.24 mbar. The table can therefore be interpreted for partial pressures of the gas mixture in air
Table 2
The table shows the partial pressures of the gases in air in relation
to the Standard pressure of dry air at sea level = 1013 mbar.
Dalton's Law
Dalton's law gives the relation between the total pressure of a gas in a container and the partial pressure of its constituents.
Note that:
The gas mixture in a closed volume does not separate. In an air-to-helium mixture, the helium does not rise to the top of the container even though it is lighter than the other gases. This is because of the thermal speed of the particles there are inter-particle collisions in the gas as well as with the container walls and therefore always a mixing process, called "diffusion"
What is Gas?
Gas is a substance, where the particles (molecules and atoms) can freely move. In thermodynamic equilibrium these particles are uniformly distributed in space, so that the pressure, partial pressures and gas composition are the same at all points of the container.
A solid is a substance, the particles are fixed in their positions, they can only vibrate and rotate. A liquid is a substance, where particles can freely slide on each other but cannot separate from each other, hindered by intermolecular forces.
What is Vapour Pressure?
When the pressure above a liquid is reduced at constant temperature, it evaporates and the resulting gas is called a vapour. This vapour, like other gases, has a pressure called vapour pressure. The vapour can become a liquid again. This is called condensation. All vapours have a saturation pressure which is the pressure of the vapour in equilibrium with its liquid. When the pressure in a system is higher than the saturation pressure, the vapour condenses, causing the pressure to return to the saturation value. Vice versa, as long as there is still a liquid in the system, the pressure cannot be reduced (by pumping) lower than the saturation pressure, because the liquid continues to evaporate.
In a container, in which a liquid and its vapour are present and the pressure is equal to the saturation vapour pressure, evaporation and condensation occur simultaneously. Water and its vapour pressure in vacuum systems need our special attention, because water is always present as humidity in the air and water is difficult to remove from vacuum systems.
We call gases vapour, when they can condense at normal temperatures. Strictly speaking all gases can condense, it depends only how much the temperature is reduced.
Vapour pressure of water at different temperatures.
Table 3
Vapour pressure of some liquids at 20° C
Table 4
What is mean free path?
The mean free path (MFP) is the average distance which a molecule of a gas- or vapour travels before it collides with another molecule. At high vacuum, these distances become very large, so the particles collide only with the walls of the vacuum chamber.
The mean free path is proportional to the pressure. At atmospheric pressure the particles collide every 10 thausandths of a millimetre, but at a pressure of 109 mbar they collide only after 68 km. The following formula gives the mean free path for air at 200C at pressure P mbar.
The mean free path for some gases at 20° C and different pressures
(in mbar)
Table 5
1 nm [Nanometer] = 10-9 m
Avogadro's Law
What is a Mole?
A Mole (abbreviated to "mol") is a unit of quantity. The definition is:
The weight of each gas in a volume of 22.415 litres (at 0°C and 1,013 bar) is equal to the relative mass of one molecule of this gas.
Example: 1 mol Helium = 22.415 litre has a weight of 4 gm.
Units of quantity can be expressed in different units:
The characterisation of an amount of gas as p x V-value is mostly used in vacuum technology.
What is a leak rate?
A leak rate is an amount of substance, which passes in a certain time through a leak. As we have seen, there are several units for quantity, so the leak rates can also be expressed in different units. The symbol for the leak rate is "q".
Normally used are:
Avogadro´s Number
The relative masses of molecules
In the past these masses have been called "atomic weight or molecular weight", but this was misleading, because these numbers are only ratios between atoms or molecules.
The relative mass of molecules M (also called molar Mass) at SI-units is defined in kg/kmol. One hypothetical standard atom has the relative mass of 1/12th of the 12 C atom. The relative mass of a Helium atom is then:
or
M = 4 g / mol
Boyle's Law
When the volume of a gas is decreased (for example by a piston), the pressure increases in the same ratio, if the temperature does not change. Compressing the volume to a half of its size doubles the pressure.
also well known as:
When a vacuum is produced and the pressure is decreased by a large amount, the gases expand to very large volumes. This occurs also with contamination on the inner walls of the vacuum chamber, which evaporates (for example fingerprints).
with equal numbers of molecules (temperature kept constant)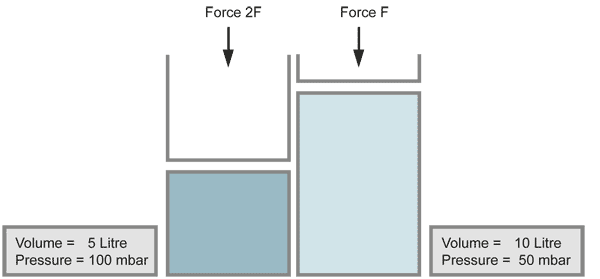
for the same number of molecules (constant temperature)
Charles' Law
The volume of a gas changes with temperature if the pressure is kept constant. When the gas becomes colder, the volume decreases. As it becomes warmer, the gas expands.
T in Kelvin (see scale of absolute temperature below).
When the temperature doubles, the volume increases similarily by the factor of 2, provided that the pressure stays constant.
with equal numbers of molecules and constant pressure
With a closer look at Charles` law, one can find a more detailed relationship between temperature and volume, known as the law of Gay-Lussac: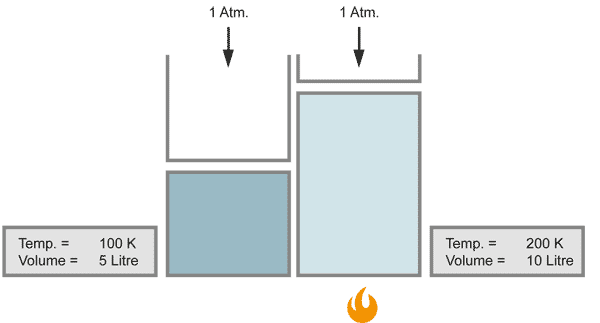
The Law of Gay-Lussac
When the temperature of a gas changes from 0°C by one degree, the volume changes by 1/273 of the original value:
or
From this law, Lord Kelvin founded the absolute scale for the temperature, in which 0 Kelvin = absolute zero = 273.15 degrees Celsius.
in Kelvin [K]
The above three law´s combine to give the universal gas law:
The universal Gas Law
Greek ν(Ny) is the symbol for the quantity in mol or kmol.
The universal gas law is not restricted like Charles´, Boyle´s and Avogadro´s laws.
The temperatures in Charles law and in the universal gas law are inserted as absolute temperatures in Kelvin.
The Universal Gas Constant
1 J (Joule) = 1 Nm = 1 Ws
For calculations, the following, equivalent values are often used:
Individual gas constants for some gases (J/kg x K)
If many calculations are made for the same gas, it is often easier to use the individual gas constant Ri
The greek alphabet
The Individual Gas Constant
If the universal gas constant is divided by the relative mass number (M) of the molecule of the gas in question, the result is the individual gas constant Ri for this gas.


